Traker Engineering Services

Laser Cutting
Our laser cutting facility can be programmed off line and run unmanned out of hours. Laser cutting provides a guaranteed quality finish with no burrs.
Continue Reading
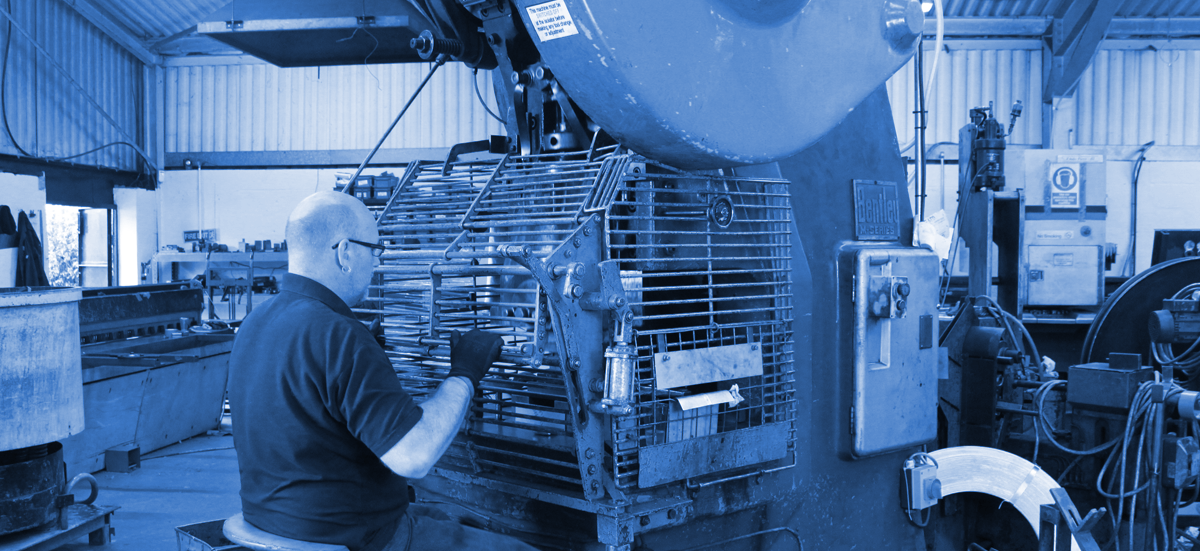
Power Press
We have a range of power presses which enables us to provide products of numerous sizes quickly and cost efficiently to various market sectors.
Continue Reading
Welding
At Traker Engineering we offer MIG, TIG and spot welding. Our fabricators are highly trained and are fully engaged in the quality control processes.
Continue Reading
Product Design
At the centre of Traker's design and engineering department is a team of highly trained competent staff ready to assist the Customer.
Continue Reading
CNC Folding
Traker Engineering has the latest press brakes. We have a large range of tooling and set ups which, creates a quick and responsive environment.
Continue Reading
CNC Punching
Traker Engineering has a fully automated high speed CNC punching cell.
Continue ReadingSample Products
Looking for a superb personalised engineering service?
REQUEST A QUOTEA few of our clients